Ethanol ( 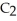
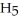 OH) melts at -114°C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/gK and 2.3 J/gK,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -125°C to liquid ethanol at -80°C?
OH) melts at -114°C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/gK and 2.3 J/gK,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -125°C to liquid ethanol at -80°C?
Definitions:
Goal Forms
The various shapes and structures that individuals' objectives and aspirations may take, influenced by personal values and societal norms.
Motivation
The internal drive or desire that propels an individual to take actions towards goals.
Faulty Cognitions
Faulty Cognitions are distorted or irrational thoughts that can lead to erroneous beliefs and negative behavior patterns.
Irrational Beliefs
Thoughts that are not based on reality or logical reasoning, often leading to negative emotions.
Q17: Which of the following statements is TRUE?<br>A)
Q35: Write a balanced reaction for which the
Q44: Give the number of core electrons for
Q66: Consider the following reaction at equilibrium.What effect
Q72: The following reaction is exothermic.Which change will
Q99: 0.300 moles of sodium nitrite are needed
Q102: Choose the bond below that is most
Q121: Which of the following statements is FALSE?<br>A)
Q124: The Lewis structure of sulfuric acid contains<br>A)
Q150: Choose the solvent below that would show