Multiple Choice
Consider the phase diagram shown.Choose the statement below that is TRUE. 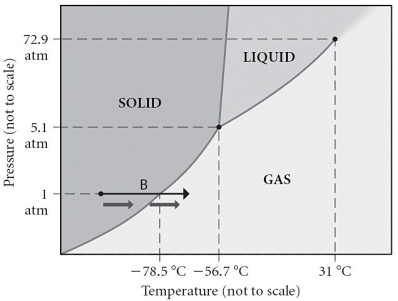
Definitions:
Related Questions
Q11: Choose the substance with the highest viscosity.<br>A)
Q16: Identify the number of electron groups around
Q17: Which of the following statements is TRUE?<br>A)
Q22: List the number of sigma bonds and
Q22: Identify the element with the smallest band
Q26: Give the direction of the reaction,if K
Q26: Soap works with water because<br>A) the polar
Q53: Which of the following compounds exhibits dipole-dipole
Q101: Which reaction below represents the second ionization
Q131: Use Lewis theory to determine the chemical