Vanadium crystallizes in a body centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 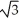 .
.
Definitions:
Thomas Friedman
is an American journalist and author known for his analysis on global issues, including globalization and the environment.
Survey Samples
Selected individuals or units from a larger population used for conducting a survey to gather data or insights.
A social media platform that allows users to post short messages, known as tweets, to share thoughts, news, or content with followers in real-time.
Research Tool
A resource or device used to assist in gathering, analyzing, and interpreting information on a specific topic.
Q18: How much heat is released when 115
Q34: What are the units of k in
Q39: How many moles of AgCl are contained
Q57: Identify the term used to describe the
Q60: How many of the following molecules have
Q80: Which of the following represents the Lewis
Q82: The decomposition of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6107/.jpg" alt="The decomposition
Q119: Express the equilibrium constant for the following
Q152: Which of the following statements is TRUE?<br>A)
Q154: The isomerization of methylisonitrile to acetonitrile CH<sub>3</sub>NC(g)→