Vanadium crystallizes in a body centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 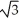 .
.
Definitions:
Financial Assets
Economic resources or assets that are paper-based, including stocks, bonds, bank deposits, and other investments.
Monetary Value
The value of a good or service determined by the amount of money that it can be traded or sold for in a market.
Demographic Characteristics
Refers to the statistical data of a population that include age, race, gender, income, migration patterns, and population growth.
Poverty Rate
The percentage of the population living below the national poverty line, reflecting those who cannot meet basic needs for food, shelter, and clothing.
Q3: Determine the value of K<sub>c</sub> for the
Q10: Consider the following reaction and its equilibrium
Q44: Given the following balanced equation,determine the rate
Q90: What is the mole fraction of oxygen
Q90: What are the units of k in
Q93: Identify the compound with the highest percent
Q105: To increase solubility of a gas into
Q111: The reaction below has a K<sub>c</sub> value
Q126: What is Δn for the following equation
Q145: Which rate law has a molecularity of