The combustion of ethylene proceeds by the reaction C2H4(g) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)
When the rate of disappearance of 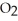 is 0.23 M s-1,the rate of disappearance of C2H4 is ________ M s-1.
is 0.23 M s-1,the rate of disappearance of C2H4 is ________ M s-1.
Definitions:
Owned Content
encompasses materials like videos, articles, and social media posts that are produced and controlled by an organization or individual for branding and marketing purposes.
Earned Media
Publicity gained through promotional efforts other than paid advertising, such as word-of-mouth, reviews, and media coverage.
Cord-cutting Trend
The trend of consumers cancelling or forgoing traditional cable or satellite television services in favor of streaming or internet-based services.
Trade Reporters
Journalists who specialize in reporting on industry-specific news, trends, and developments, catering to professionals within those sectors.
Q25: Place the following substances in order of
Q33: Identify the element with the largest band
Q60: Give the coordination number for a face-centered
Q86: How many milliliters of 0.120 M NaOH
Q87: 100.0 mL sample of 0.10 M NH<sub>3</sub>
Q89: Define triple point.<br>A) the temperature, pressure, and
Q122: What is the hydronium ion concentration of
Q128: Choose the molecule or compound that exhibits
Q158: Which of the following is a Lewis
Q183: What is the pH of a solution