The combustion of ethylene proceeds by the reaction C2H4(g) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)
When the rate of disappearance of 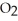 is 0.23 M s-1,the rate of disappearance of C2H4 is ________ M s-1.
is 0.23 M s-1,the rate of disappearance of C2H4 is ________ M s-1.
Definitions:
Proportionately Adjusted Balance Sheet
A balance sheet method that adjusts the assets, liabilities, and equity based on a company's percentage ownership in other entities.
Contractual Agreement
A contractual agreement is a legally binding contract between two or more parties, outlining the terms and conditions of an arrangement.
Equity Method
An accounting technique used by firms to assess the profits earned by their investments in other companies.
Unrealized Gains and Losses
Increases or decreases in the value of investments that have not been sold, thus not yet resulting in actual profit or loss.
Q13: Which of the following is considered a
Q29: When two waves interact with the crests
Q36: Identify for the rhombohedral unit cell.<br>A) a
Q50: A molecule containing a central atom with
Q56: Given the following rate law,how does the
Q103: How much energy must be removed from
Q112: Give the direction of the reaction,if K
Q115: Fresh vegetables with high water content do
Q123: An important buffer in the blood is
Q126: _ is found in carbonated beverages due