For the first-order reaction,2 N2O(g) → 2 N2(g) + O2(g) ,what is the concentration of N2O after 3 half-lives if 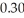 mol of N2O is initially placed into a 1.00-L reaction vessel?
mol of N2O is initially placed into a 1.00-L reaction vessel?
Definitions:
Competition Act
Legislation designed to prevent anti-competitive practices, promote and maintain fair competition in the market.
Criminal Law
The body of law pertaining to crimes and punishment, regulating the conduct of individuals and imposing penalties for unlawful acts.
Loss Leader
A pricing strategy where a product is sold at a loss to attract customers, with the expectation that those customers will make additional purchases of more profitable items.
False Advertising
The act of misleading consumers through deceptive, incorrect, or unsubstantiated claims about a product or service.
Q12: The equilibrium constant is given for one
Q27: List the compound in marble that is
Q55: Determine the pH of a 0.00598 M
Q66: Determine the pOH in a 0.235 M
Q84: Identify the rate-determining step.<br>A) the slowest step<br>B)
Q97: How many of the following molecules contain
Q108: The decomposition of dinitrogen pentoxide is described
Q116: The following reaction is exothermic.Which change will
Q134: Which of the following is a weak
Q147: Identify the indicator that can be used