At 900.0 K,the equilibrium constant (Kp) for the following reaction is 0.345. 2 SO2(g) + O2(g) ⇌ 2 SO3(g)
At equilibrium,the partial pressure of SO2 is 35.0 atm and that of 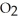 is 15.9 atm.The partial pressure of SO3 is ________ atm.
is 15.9 atm.The partial pressure of SO3 is ________ atm.
Definitions:
Affection
A feeling of fondness or tenderness toward someone or something.
Prohibitive
Referring to something that discourages action or imposes a constraint by rules, laws, or circumstances.
Restrictive
Imposing limitations or restrictions on actions, movements, or decisions.
Affectionate
Exhibiting feelings of fondness or tenderness towards others.
Q8: Identify the characteristics of a face-centered cubic
Q15: What can change the direction of a
Q73: Place the following aqueous solutions of nonvolatile,nonionic
Q84: Identify the compound with the highest boiling
Q84: Given the following equation, H<sub>2</sub>O(g)+ CO(g)→ H<sub>2</sub>(g)+
Q91: The fluorocarbon C<sub>2</sub>Cl<sub>3</sub>F<sub>3</sub> has a normal boiling
Q112: Assign the appropriate labels to the phase
Q129: In aqueous solution,hypobromite ion,BrO<sup>-</sup>,reacts to produce bromate
Q137: A strong electrolyte _ in solution.<br>A) precipitates<br>B)
Q140: What is the molar solubility of AgCl