The pH of an aqueous solution at 25.0°C is 10.66.What is the molarity of 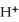 in this solution?
in this solution?
Definitions:
Rate Of Formation
The speed at which a product is created in a chemical reaction, usually measured in moles per liter per second.
High-energy Particles
Particles that possess energy significantly greater than average, often referring to subatomic particles such as protons, neutrons, and cosmic rays.
Accumulated Degree Hours
A measurement unit for the thermal energy accumulated, used in forensic entomology to estimate the time since death based on insect development.
Chemiluminescence
The emission of light (luminescence) as the result of a chemical reaction without the need for external light sources.
Q2: Give the equation for a saturated solution
Q29: What is △n for the following equation
Q43: Identify the weak monoprotic acid.<br>A) H<sub>2</sub>CO<sub>3</sub><br>B) NaOH<br>C)
Q88: A solution contains 0.021 M Cl⁻ and
Q98: The decomposition of dinitrogen pentoxide is described
Q107: What is the pH of the resulting
Q112: What is the molality of a D-mannose
Q138: What element is being oxidized in the
Q152: Determine the [OH⁻] concentration in a 0.235
Q160: The base-dissociation constant of ethylamine (C<sub>2</sub>H<sub>5</sub>NH<sub>2</sub>)is 6.4