What is the pH of a solution made by mixing 30.00 mL of 0.10 M acetic acid with 50.00 mL of 0.100 M KOH? Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for 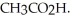
Definitions:
Carbon Dioxide
A gas characterized by its lack of color and smell, produced when carbon and organic compounds burn, and during the respiration process, which plants absorb in photosynthesis.
Definite Proportions
A principle stating that a chemical compound always contains its component elements in fixed ratio by mass and does not depend on its source and preparation method.
Relative Masses
A concept referring to how the mass of one particle or object compares to another, often used in chemistry to discuss the atomic mass of elements relative to the carbon-12 standard.
Dalton's Atomic Theory
A fundamental theory proposed by John Dalton in the early 19th century, postulating that elements are composed of indivisible and indestructible atoms which combine in chemical reactions to form compounds.
Q1: Carbon dioxide combines with rainwater to form<br>A)
Q6: Write the balanced chemical equation for the
Q38: Consider the following reaction,equilibrium concentrations,and equilibrium constant
Q61: Predict the species that will be reduced
Q68: Consider the following reaction,equilibrium concentrations,and equilibrium constant
Q74: Identify the radiotracer used to for PET
Q81: An exothermic reaction is a process that<br>A)
Q91: The following reaction represents what nuclear process?
Q97: What is true if ln K is
Q134: The following reaction is exothermic.Which change will