How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten 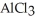 with an electrical current of 12.0 A?
with an electrical current of 12.0 A?
Definitions:
Math Instruction
A command or operation in computer programming and digital logic that performs mathematical calculations.
Logically True
A condition or statement that is valid or correct according to logical or Boolean principles.
SUB Instruction
An operation in programming and computing that subtracts one value from another or more values and returns the result.
Shipped Parts
Components or items that have been packaged and dispatched to a destination as part of a delivery or supply chain process.
Q1: Identify the metal that is a noble
Q2: Identify the radioactive element that is so
Q10: Which of the following describes a primary
Q15: Identify a property of fluorine.<br>A) reacts with
Q17: What is the pH of a 0.30
Q32: Determine the [OH<sup>-</sup>] concentration of a 0.123
Q73: Which of the following is a weak
Q83: Which of the following is a Lewis
Q100: Determine which of the following pairs of
Q109: What is the pH of a solution