Which one of the statements below is concerning the following reaction:
NH3(g) + H2O 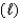 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
Definitions:
Forgone Entrepreneurial Income
The potential earnings that an entrepreneur sacrifices by choosing to operate their business instead of working elsewhere for a salary.
Production Costs
Expenses incurred in the manufacturing or production process, including raw materials, labor, and overhead costs.
Average Product
The output produced per unit of input, calculated by dividing total output by the total number of units of the input used.
Nonlabor Resources
Resources used in the production of goods and services that do not involve labor, such as capital, land, and raw materials.
Q3: A $1000, 6% bond redeemable at par
Q3: A chemical reaction in a bomb
Q18: A company has two investment choices. Alternative
Q32: Which of the following are extensive properties:
Q45: How many electrons can be described by
Q50: What base results when Li<sub>2</sub>O reacts with
Q50: If the de Broglie wavelength of
Q58: If a gallon (3.78 L)of latex
Q67: Which one of the following molecules is
Q72: Which of the following statements is/are CORRECT