Aspirin (C9H8O4) is produced by the reaction of salicylic acid (M = 138.1 g/mol) and acetic anhydride
(M = 102.1 g/mol) .C7H6O3(s) + C4H6O3( 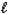 ) C9H8O4(s) + C2H4O2(
) C9H8O4(s) + C2H4O2( 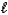
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol) can theoretically be obtained?
Definitions:
Detecting Pain
The physiological and psychological process by which organisms recognize and respond to stimuli that are noxious and potentially harmful.
Jnd
The minimum difference in stimulation that a person can detect 50% of the time, also known as the just noticeable difference.
Absolute Threshold
The minimum intensity level of a stimulus required for a person to detect its presence at least half the time.
Proportional Differences
Variations that are relative to the size or significance of the items being compared.
Q17: What is the change in internal
Q22: A 45.0 L gas cylinder contains
Q34: An aqueous nitric acid solution has a
Q46: Which of the following statements is/are
Q50: The density of a particular solid
Q52: Which of the following statements concerning molecular
Q55: If a molecule has a positive and
Q67: The procedure by which electrons are assigned
Q75: Which of the following compounds is
Q90: Which of the following elements is not