If 0.1800 g of impure soda ash (Na2CO3) is titrated with 15.66 mL of 0.1082 M HCl,what is the percent purity of the soda ash?
Na2CO3(aq) + 2 HCl(aq) 2 NaCl(aq) + H2O( 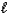 ) + CO2(g)
) + CO2(g)
Definitions:
Measurement Problems
issues or challenges encountered in the process of quantifying variables or phenomena for research or analysis.
Effectiveness
The degree to which something achieves its intended outcome or purpose, often used in business to evaluate the success of strategies or products.
Television Commercials
Short marketing messages broadcasted on television to promote products, services, or ideas to a wide audience.
Response Bias
A tendency of respondents to answer questions on a survey untruthfully or misleadingly, often to present themselves in a favorable light or due to misunderstanding.
Q6: What is the total volume of
Q10: The introduction of a new product requires
Q16: Which of the following molecules or ions
Q40: The temperature of a specific amount of
Q49: Which of the following is the
Q51: Ionic and molecular compounds that form ions
Q52: What is the wavelength of a photon
Q57: Which of the following atoms contains the
Q74: Dry ice converts directly from a solid
Q88: A vessel with a volume of 26.9