If 0.1800 g of impure soda ash (Na2CO3) is titrated with 15.66 mL of 0.1082 M HCl,what is the percent purity of the soda ash?
Na2CO3(aq) + 2 HCl(aq) 2 NaCl(aq) + H2O( 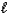 ) + CO2(g)
) + CO2(g)
Definitions:
Activity-Based Costing
An accounting method that assigns overhead and indirect costs to related products and services by identifying cost drivers.
Machine-Hours
Machine-Hours measure the amount of time machines are in operation during a certain period, often used for allocating machine-related expenses to products.
Activity Cost Pools
A method in cost accounting where costs are accumulated according to the activities that incur them.
Activity-Based Costing
A costing methodology that assigns costs to products and services based on the resources consumed in the production process.
Q3: What is the total number of electrons
Q24: What is the smallest whole number
Q29: Which of the following is the
Q38: What is the formal charge on each
Q49: How many bromide ions are in
Q52: When 0.5000 grams of an unknown
Q56: What type of orbital is designated n
Q59: If a cordless phone operates at
Q67: What is the value of the orbital
Q82: What is the symbol for an ion