The thermochemical equation for the combustion of methanol is shown below.
CH3OH( 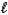 ) + 3/2 O2(g) CO2(g) + 2 H 2O(g)
) + 3/2 O2(g) CO2(g) + 2 H 2O(g)
rH = -638.7 kJ/mol-rxn
What is the enthalpy change for the combustion of 8.59 g CH3OH?
Definitions:
Authorization Cards
Documents signed by employees to indicate their wish to be represented by a union, often used in the process of forming a union within a workplace.
Collective Agreement
A written contract negotiated between an employer and a union representing the employees, outlining terms of employment.
Union Shop
Provision of the collective agreement that requires employees to join the union as a condition of their employment.
Compulsory Unionization
A policy or practice requiring all employees in a particular sector or company to become members of a union.
Q13: Which of the following is a correct
Q17: What is the formal charge of
Q33: What are the smallest whole number
Q34: Bismuth(III)sulfide is an ionic compound formed from
Q36: A company spends $117 500 today and
Q41: The Radium Hot Springs plans to install
Q45: How many electrons can be described by
Q46: Which of the following statements is/are
Q65: At STP,as the molar mass of the
Q70: Which of the following atoms is diamagnetic