The thermochemical equation for the combustion of butane is shown below.C4H10(g) + 13/2 O2(g) 4 CO2(g) + 5 H2O( 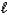 )
)
rH = -2877 kJ/mol-rxn
What is the enthalpy change for the following reaction?
8 CO2(g) + 10 H2O( 11ea8937_ab5c_9838_a16d_1fd5f2335456_TB4499_11 ) 2 C4H10(g) + 13 O2(g)
Definitions:
Used
Previously owned or utilized by someone before its current owner or application.
Sales Supplies
Items and materials used in the process of selling goods or services.
Used
Items that have been previously owned or utilized by someone before being sold or transferred.
Selling and Administrative Expense
Costs unrelated to direct production of goods or services, including costs associated with sales, marketing, and general administration.
Q17: What is the formal charge of
Q31: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q38: As pure molecular solids,which of the following
Q45: Internal energy and enthalpy are state functions.What
Q53: What is the oxidation number of each
Q56: A 3.361 g sample of a hydrocarbon
Q56: Which element has the ground state electron
Q56: Which molecule will have the following valence
Q66: If 50.0 g of benzene,C<sub>6</sub>H<sub>6</sub>,at 25.0
Q83: A neutral atom of the isotope <sup>197</sup>Au