What quantity,in moles,of oxygen is consumed when 369.3 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 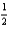
O2(g) H2O(l) ; rH° = -285.8 kJ/mol-rxn
Definitions:
Transnational Collaboration
The practice of individuals, groups, or entities from different nations working together toward a common goal or on a shared project.
Labor Movement
A broad social movement seeking to improve rights, wages, and working conditions for workers through collective action and labor unions.
Multinational Corporations
Companies that operate in several countries around the world, managing production or delivering services in more than one country.
Labor Standards
Regulations and laws that establish the minimum requirements for working conditions, including hours of work, wages, safety, and health protections for workers.
Q1: What is the correct name for Cl<sub>2</sub>O<sub>7</sub>?<br>A)
Q5: Refer to Diagram 9-1.According to molecular orbital
Q7: Which of the following is a representation
Q12: Transmittance,T,is defined as the intensity of light
Q24: What is the smallest whole number
Q28: What is the molar mass of sodium
Q42: Hydrazine,N<sub>2</sub>H<sub>4</sub>,is a liquid used as a
Q61: Which one of the following sets of
Q72: Two students independently determine the volume of
Q73: The volume of a carbon atom