Using the Following Thermochemical Data:
Calculate rH for the Following Reaction:
HoF3(s)+ 3HCl(g)
Using the following thermochemical data: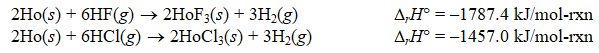
Calculate rH for the following reaction:
HoF3(s) + 3HCl(g) HoCl3(s) + 3HF(g)
Definitions:
Competitive Firms
Companies that operate in a market where there are many buyers and sellers, such that no single entity can dictate the market price of goods and services.
Price
The monetary value that must be paid to secure a good or service.
Product
Anything that can be offered to a market to satisfy a want or need, including physical goods, services, experiences, events, persons, places, properties, organizations, information, and ideas.
Total Revenues
The total income generated from the sale of goods or services before any costs are subtracted.
Q15: Which species is reduced in the
Q16: A company needs to spend $15000 for
Q29: Project A requires an immediate investment of
Q29: Which of the following is a
Q34: Use the bond energies provided to complete
Q41: The Radium Hot Springs plans to install
Q57: Which of the following atoms contains the
Q65: When 0.236 mol of a weak
Q66: The change in energy for the
Q105: Silver has two stable isotopes with masses