Determine the standard enthalpy of formation of calcium carbonate from the thermochemical equations given below.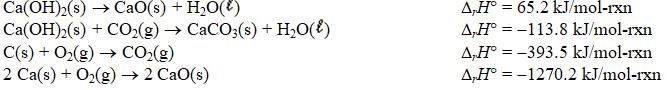
Definitions:
Direct Product Cost
Costs that can be directly attributed to the production of specific goods or services, including materials and labor.
Advertising Campaign
An advertising campaign is a series of advertisement messages that share a single idea and theme which make up an integrated marketing communication (IMC).
Chocolate Covered Almonds
A confectionery product consisting of almonds coated in chocolate, often served as a sweet snack.
Manufacturing Costs
Expenses directly associated with the production of goods, including materials, labor, and factory overhead.
Q11: What kind of change is depicted below?
Q29: How much heat is liberated at
Q41: The Radium Hot Springs plans to install
Q53: How many sigma and pi bonds are
Q57: Which of the following atoms contains the
Q69: Which of the following is the correct
Q73: For an ideal gas, which two variables
Q79: In which of the following reactions
Q87: Use VSEPR theory to predict the molecular
Q94: When both of the electrons in a