When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) according to the following equation,98.8 kJ of energy are absorbed.
Fe2O3(s) + 3 H2(g) 2 Fe(s) + 3 H2O(g)
(A) 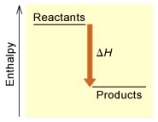
(B) 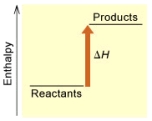
Is the reaction endothermic or exothermic,and which of the enthalpy diagrams above
Represents this reaction?
Definitions:
Regional Purchasing Offices
Offices located in various geographical regions tasked with buying goods and services for a company, optimizing logistics and reducing costs.
International Trade
The exchange of goods, services, and capital between countries and territories.
Overall Value
The total worth or benefit derived from a product, service, or activity, considering all factors.
Lower Price
A strategy or situation where the cost of goods, services, or commodities is reduced to make them more attractive to buyers.
Q7: What is the IRR for the following
Q8: A _,designated by the Greek symbol
Q24: The Bohr model predicts that the energy
Q30: How many p orbitals are in the
Q44: Which of the following processes will result
Q46: How many nutritional calories are equivalent
Q54: The hybridization of the nitrogen atom in
Q57: Which of the following boils at
Q89: The pressure of 5.4 L of nitrogen
Q92: Into a 2.22-liter container at 25°C are