What quantity,in moles,of oxygen is consumed when 369.3 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 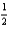
O2(g) H2O(l) ; rH° = -285.8 kJ/mol-rxn
Definitions:
Promotion
The activities that involve all the ways in which companies tell their customers about their offering.
Designer Cookware
High-quality cooking utensils and equipment typically created by or associated with known designers, often featuring unique styles or functionalities.
4 Ps Model
A marketing framework that divides the marketing process into four components: Product, Price, Place, and Promotion.
Harvard Business School
An elite private business school in Boston, Massachusetts, known for its influential MBA program and extensive case method teaching.
Q9: Which of the following observations is/are
Q17: What evidence does the photoelectric effect provide
Q26: Refer to Diagram 9-1.According to molecular orbital
Q45: Which of the following statements concerning the
Q51: The complete electron configuration of tin is<br>A)
Q57: Which of the following boils at
Q61: What is the oxidation number of each
Q63: The element chlorine has two stable isotopes,chlorine-35
Q65: Which of the following cations has the
Q105: Silver has two stable isotopes with masses