Determine the standard enthalpy of formation of Fe2O3(s) given the thermochemical equations below.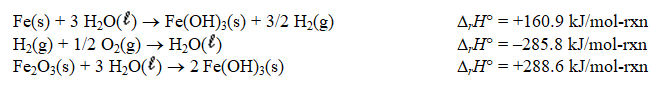
Definitions:
Gandhi
An influential leader and the preeminent figure of the Indian independence movement, known for his philosophy of non-violent resistance.
Styles Of Leadership
Different approaches and methods by which people lead others, including autocratic, democratic, and laissez-faire styles.
Assessment Instrument
A tool or method used to evaluate, measure, or appraise a particular characteristic, ability, or quality in individuals or groups.
Leadership Ability
The capacity to guide, direct, or influence people in a way that achieves goals and garners respect and cooperation from others.
Q2: Which one of the following statements is
Q4: One statement of the first law of
Q5: Which of the following orbital boundary surfaces
Q9: What is the correct Lewis structure for
Q15: The overall chemical equation resulting from
Q27: If two or more species have the
Q28: How many values are there for the
Q41: A light emitting diode (L.E.D.)emits photons with
Q65: What is the mass percent of
Q77: Predict which ionic compound has the highest