Using the Following Thermochemical Data:
Calculate rH for the Following Reaction:
HoF3(s)+ 3HCl(g)
Using the following thermochemical data: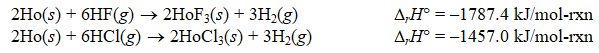
Calculate rH for the following reaction:
HoF3(s) + 3HCl(g) HoCl3(s) + 3HF(g)
Definitions:
Radioactive Isotopes
Variants of chemical elements that decay over time, releasing radiation, and are used in medical diagnosis, treatment, and research.
Metabolic Activity
The sum of all biochemical processes that occur within an organism, including those that break down nutrients for energy and those that build up new substances.
Cerebral Voltammetry
Technique used to identify the concentration of specific chemicals in the brain as animals behave freely.
Neurotransmitter Levels
The concentration of chemical messengers in the nervous system that transmit signals across synapses from one neuron to another.
Q7: Non-ideal behavior for a gas is most
Q12: Tamia Industries plans to replace the outdated
Q13: As the standard deviation of an average
Q23: What is the hybridization of an
Q48: An element consists of three isotopes.The abundance
Q51: Sulfur hexafluoride is produced by reacting
Q51: Ammonia,NH<sub>3</sub>,is used as a refrigerant.At its
Q52: Use Lewis structures to predict the bond
Q59: What is the hybridization of the sulfur
Q70: In the Lewis formula for sulfur dioxide,SO<sub>2</sub>,the