When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) according to the following equation,98.8 kJ of energy are absorbed.
Fe2O3(s) + 3 H2(g) 2 Fe(s) + 3 H2O(g)
(A) 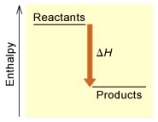
(B) 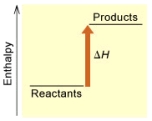
Is the reaction endothermic or exothermic,and which of the enthalpy diagrams above
Represents this reaction?
Definitions:
Multivitamin Preparations
Products that contain a combination of vitamins, minerals, and other nutritional elements, intended to supplement the diet.
Guillain-Barré Syndrome
An autoimmune disorder in which the body’s immune system attacks the nerves, leading to muscle weakness and paralysis.
Telemetry Monitor
A medical device used to remotely monitor a patient's vital signs (e.g., heart rate, oxygen level) in real-time.
Respiratory Efforts
The act of breathing, encompassing inhalation and exhalation, vital for oxygen exchange.
Q3: Absolute zero is the point at
Q5: Refer to Diagram 9-1.According to molecular orbital
Q6: What is the correct name for Ca(CH<sub>3</sub>CO<sub>2</sub>)<sub>2</sub>?<br>A)
Q13: What volume of 2.52 M HCl
Q17: What is the change in internal
Q33: Which of the following statements is INCORRECT?<br>A)
Q33: A particular compound has an enthalpy of
Q54: The hybridization of the nitrogen atom in
Q55: A linear relationship exists between the natural
Q63: A sample of helium gas occupies 14.7