Which of the following statements is/are CORRECT?
1) The angular momentum quantum number, 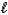
,of an electron in an s orbital is always equal to 0.
2) The magnetic quantum number, 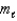
,of an electron in a p orbital is +1,0,or -1.
3) The principle quantum number,n,of an electron in a d orbital must be equal to or greater than 3.
Definitions:
Work Environments
Refers to the setting, conditions, and culture in which an individual performs their job.
Expected Outcomes
Anticipated results or consequences of an action or decision, often based on previous experiences or statistical forecasts.
Social Cognitive Theory
A framework for understanding, predicting, and changing human behavior that emphasizes the interplay between personal factors, behaviors, and environmental influences.
Competence
The ability or skill to perform a task effectively and efficiently.
Q1: Chromium (atomic mass 52.00 g/mol)crystallizes in a
Q17: Of the following,the only empirical formula is<br>A)
Q19: What is the <span class="ql-formula"
Q24: What is the distance,in atomic radii,along
Q25: Which of the labeled carbon atoms (C<sub>1</sub>-C<sub>4</sub>)is/are
Q37: What is the distance,in atomic radii,across
Q43: What is responsible for capillary action,a property
Q44: A metal crystallizes in a face-centered cubic
Q53: The pressure exerted by gas molecules inside
Q68: What is the bond angle in a