One way to isolate metals from their ores is to react the metal oxide with carbon as shown in the following reaction (M = metal) : 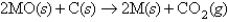
If 34.14 g of a metal oxide reacted with excess carbon and 4.38 L of CO2 formed at 100°C and 1.50 atm,what is the identity of the metal?
Definitions:
Q6: Which of the following electron configurations corresponds
Q11: Place the following cations in order from
Q16: Which of the following statements is/are CORRECT?<br>1)Specific
Q35: Magnesium sulfide (molar mass 56.37 g/mol)has a
Q39: For a dilute solution of NH<sub>4</sub>NO<sub>3</sub>,the van't
Q53: A 100 g sample of each
Q55: What is the pH of 5.3
Q78: At 75.0 <sup> <span class="ql-formula" data-value="\circ"><span
Q86: Which are the Brønsted-Lowry acids in the
Q90: What is the correct Lewis structure for