Multiple Choice
On the phase diagram below,which point corresponds to conditions where solid,liquid,and gas phases all exist? 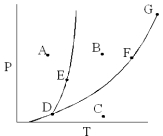
Definitions:
Related Questions
Q16: Carbonic acid is a diprotic acid,H<sub>2</sub>CO<sub>3</sub>,with
Q29: A low melting solid readily dissolves in
Q35: To make a buffer with a pH
Q48: Carbon monoxide reacts with oxygen to
Q56: Which molecule will have the following valence
Q74: Given the following,determine <span class="ql-formula"
Q78: Which atom has the ground state electronic
Q81: The solubility of copper(II)oxalate is 3.2
Q81: When the pressure of an equilibrium mixture
Q82: Which of the following equations corresponds to