What is the overall order of the reaction below
NO(g) + O3(g) NO2(g) + O2(g)
If it proceeds via the following rate expression? 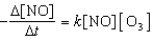
Definitions:
Direct Labor Hours
The sum of hours dedicated by employees directly linked to producing products or offering services.
Manufacturing Costs
Manufacturing costs refer to the total expenses involved in the process of producing goods, including direct materials, direct labor, and manufacturing overhead.
Journal Entries
The method used in bookkeeping to record all the financial transactions of a business.
Raw Materials
Fundamental substances used in the creation or manufacturing of goods.
Q1: What is the half-life of a
Q2: Which of the following molecules is expected
Q25: What is <span class="ql-formula" data-value="\Delta"><span
Q45: Which of the following concerning the behavior
Q48: What is the molecular geometry around a
Q54: Which of the following ionic compounds is
Q57: In any chemical process,energy must be conserved.This
Q68: The Arrhenius equation, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="The Arrhenius
Q73: _ is a measure of the degree
Q74: At 800 K,the equilibrium constant,K<sub>p</sub>,for the