Which of the following conclusions concerning the concentration-time plot provided below is/are correct?
1) The concentration of substance D is decreasing over time.
2) The instantaneous reaction rate at point A is less than the instantaneous reaction rate at point B.
3) Substance D is a product of the reaction. 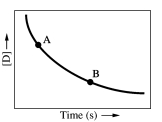
Definitions:
Electromagnetic Spectrum
The range of all types of electromagnetic radiation, including gamma rays, X-rays, ultraviolet light, visible light, infrared light, microwaves, and radio waves, each varying in wavelength and energy.
Electromagnetic Radiation
A form of energy that is propagated through space or matter, including radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays.
Absorption
The process by which one substance takes up or takes in another substance, at the molecular or ionic level.
Frequency
The number of occurrences of a repeating event per unit of time, often used in the context of waves (e.g., sound waves, electromagnetic waves).
Q7: What is the pH of a
Q18: When a weak base is titrated with
Q38: Elements that have their highest energy
Q38: A 50.00-mL solution of 0.0729 M
Q42: The nitrogen atom in cyanide ion,CN<sup>-</sup>,is surrounded
Q45: If a catalyst is present in a
Q47: Which of the following species is amphiprotic
Q47: If 25 mL of 0.750 M HCl
Q55: The bandgap of Si is 107.1
Q64: The pre-exponential,A,in the Arrhenius equation is called