What is the overall order of the reaction below
NO(g) + O3(g) NO2(g) + O2(g)
If it proceeds via the following rate expression? 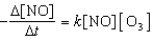
Definitions:
Web Services
Software systems designed to support interoperable machine-to-machine interaction over a network.
MIS Professionals
Individuals specializing in Management Information Systems, focusing on the study of people, technology, organizations, and the relationships among them.
On-Premise Computing
A model of IT management in which companies own their IT infrastructure (their software, hardware, networks, and data management) and maintain it in their data centers.
Business Environments
The combination of all internal and external factors that influence a company's operating situation, including customers, competitors, stakeholders, suppliers, industry trends, regulations, and other external forces.
Q8: Which of the following pure liquids is
Q10: What is the pH of a
Q12: The metal barium crystallizes in a body-centered
Q26: Which ionic compound forms a pH-neutral
Q29: For which of the following substances is
Q35: The volume of a sample of gas
Q36: On the phase diagram below,which point corresponds
Q71: What is the electron-pair geometry around an
Q75: What is the formal charge on carbon
Q78: A buffer contains 0.50 mol NH<sub>4</sub><sup>+</sup> and