The reaction quotient,Q,for a system is 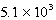 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 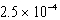
,what will happen as the reaction mixture returns to equilibrium?
Definitions:
Regression Stage
A phase of development or transition characterized by a return to earlier patterns of behavior, often as a response to stress or trauma.
Trickle-across Effect
A trend diffusion process where fashion and ideas move horizontally within similar social levels or groups.
Class Structure
The organization of society into hierarchies based on socioeconomic status, class, or other distinctions.
Fashion Acceptance Cycle
The process through which new fashion trends are introduced, rise in popularity, peak, and eventually decline.
Q5: Which of the following units are consistent
Q11: The phase diagram for CO<sub>2</sub> has
Q20: Which ion occurs in the highest concentration
Q21: In a first-order reaction,the half-life is
Q25: Which of the following might be used
Q36: For the equilibrium PCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q60: Which two of the following materials are
Q66: What is the general valence shell electron
Q84: Given the initial rate data for
Q92: Into a 2.22-liter container at 25°C are