For the reaction TlSCN(s)  Tl+(aq) + SCN-(aq) ,Kc =
Tl+(aq) + SCN-(aq) ,Kc = 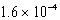
At 25 C.Which of the following concerning a 125 mL solution containing 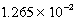
M Tl+, 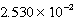
M SCN- and a large excess of TlSCN(s) is/are correct?
1) The mixture is at equilibrium.
2) Additional TlSCN(s) must precipitate to attain equilibrium.
3) The reaction quotient (Q) is greater than one.
Definitions:
Q11: The density of a gas is 1.96
Q13: Which of the following statements is/are CORRECT?<br>1)Of
Q24: Suppose 50.00 mL of 2.0
Q32: In which reaction is <span
Q36: Which of the following statements concerning
Q40: Which of the following is not responsible
Q47: Which of the following species is amphiprotic
Q57: In any chemical process,energy must be conserved.This
Q66: What is the general valence shell electron
Q83: Which of the following species is the