Nitrogen trifluoride decomposes at to form nitrogen and fluorine gases according to the following equation: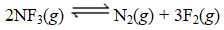
6.00-L reaction vessel is initially charged with 1.96 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0380 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L.atm.mol.K)
Definitions:
Pre-Emptive Right Provision
A clause usually found in a company's charter or bylaws that gives existing shareholders the first opportunity to buy new shares before they are offered to the public.
Authorized Capital
The maximum amount of share capital that a company is authorized by its corporate charter to issue to shareholders.
Treasury
A government department responsible for managing the financial resources, including revenue collection and expenditure.
Corporation Not Bound
Indicates a scenario where a corporate entity is not legally obligated or tied to an agreement or contract.
Q3: The vapor pressure of water at 90°C
Q9: If <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q14: What is the solution pH when
Q28: What is the mole fraction of urea,CH<sub>4</sub>N<sub>2</sub>O,in
Q39: Which of the following is/are physical properties
Q49: According to the Brønsted-Lowry definition,an acid<br>A) increases
Q55: A linear relationship exists between the natural
Q59: What is the hydroxide-ion concentration in
Q63: The standard free energy change for
Q69: The decomposition of phosphine,PH<sub>3</sub>,follows first-order kinetics.<br>4