When 0.20 mole HF is dissolved in water to a volume of 1.00 L,5.8% of the HF dissociates to form F-(aq) .What is the equilibrium constant for the reaction?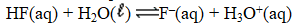
Definitions:
Blisspoint
The optimal level of consumption or exposure where consumer satisfaction is maximized, often used in context with food flavors or media content.
Delay-Reduction Theory
Behavior economic theory that states that overall behavior in a choice task is based on the matching law, while individual choices are determined by which choice produces the shortest delay in the next reinforcement
Matching Law
A principle in behavioral psychology stating that the proportion of responses on a particular schedule matches the proportion of reinforcers obtained on that schedule.
Maximization Theory
The principle that decision-makers aim to make choices that result in the highest possible benefit or utility.
Q11: What is the vapor pressure at 20°C
Q11: Place the following cations in order from
Q15: Diluting concentrated sulfuric acid with water
Q16: Silver chloride crystallizes with the sodium chloride
Q27: Rubidium iodide (molar mass 212.4 g/mol)has a
Q28: Many homes are heated using natural gas.The
Q37: Hydrazine,N<sub>2</sub>H<sub>4</sub>,is produced by the Raschig process-the oxidation
Q64: Which is the best colored indicator
Q81: The density of H<sub>2</sub> gas in
Q86: A certain person has a body temperature