Assume that the following chemical reaction is at equilibrium.
C(s) + H2O(g) 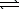 H2(g) + CO(g)
H2(g) + CO(g)
Which of the following statements is/are CORRECT?
1) Increasing the concentration of H2(g) will cause the reaction to proceed in the backward direction,increasing the equilibrium concentration of H2O(g) .
2) Decreasing the temperature will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g) .
3) Increasing the amount of C(s) will cause the reaction to proceed in the forward direction,increasing the equilibrium concentration of CO(g) .
Definitions:
ABC System
Activity-Based Costing; a method of assigning overhead and indirect costs to specific products or projects, based on their usage of activities.
Single-Base Allocation
A cost allocation method where overhead costs are distributed based on a single criterion or cost driver.
Cost Difference
The variance between the actual cost and the standard or expected cost of an item or activity.
ABC System
Activity-Based Costing System, a method of assigning overhead and indirect costs to specific products and services based on their consumption of resources.
Q5: What intermolecular force(s)is/are present in solid NH<sub>3</sub>?<br>1)induced
Q8: For a certain reversible process q
Q25: For the zero-order reaction below,a graph
Q29: The K<sub>sp</sub> of BaSO<sub>4</sub> is 1.1
Q40: Which of the following is not responsible
Q47: Metal hydrides are used to remove
Q68: A 50.0 mL sample of 0.155
Q69: Write the expression for K<sub>p</sub> for the
Q75: What is the total number of valence
Q83: If a reaction is third-order with respect