The thermochemical equation for the formation of ammonia from elemental nitrogen and hydrogen is as follows.
N2(g) + 3 H2(g) 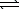 2 NH3(g) H = -92.2 kJ
2 NH3(g) H = -92.2 kJ
Given a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
Definitions:
Coaches
Professionals who provide guidance and training to improve an individual's skills, performance, or personal development.
Competing Values Framework
A model used to assess organizational culture and effectiveness, which identifies four underlying values that compete with each other: internal focus and integration vs. external focus and differentiation, and flexibility and discretion vs. stability and control.
Adhocracy Culture
An organizational culture characterized by flexibility, employee empowerment, and an emphasis on innovation, often seen in dynamic and creative industries.
Flexibility
The quality of being able to adapt to new, different, or changing requirements.
Q11: What is the vapor pressure at 20°C
Q19: What is the K<sub>c</sub> equilibrium-constant expression for
Q21: Given the following equilibria,<br>Ni<sup>2+</sup>(aq)+ 2 OH<sup>-</sup>(aq)
Q24: A molecule that can behave as either
Q43: Which relationship correctly compares the rates
Q48: Calculate the pH of a 0.04
Q49: Use the following thermodynamic data<br>Species<br><br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q56: Molten Al<sub>2</sub>O<sub>3</sub> is reduced by electrolysis
Q66: What is the general valence shell electron
Q79: What is the pH of a