Ozone is formed from oxygen.3 O2(g) 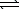 2 O3(g)
2 O3(g)
Calculate the value of Kp,given that Kc = 2.5 10-29 at 298 K.(R = 0.08206 L.atm/mol.K)
Definitions:
Nonaromatic
Refers to compounds that do not possess a ring structure with alternating single and double bonds, lacking the characteristic stability of aromatic systems.
Nonaromatic
Refers to organic compounds that do not exhibit the stability associated with aromatic rings, often lacking delocalized pi-electron systems.
[10]annulene
A ten carbon ring compound with alternating single and double bonds, known for its unusual properties and contribution to the study of aromaticity.
Aromatic
A class of cyclic, planar compounds with electrons delocalized over the entire structure, providing unique stability, examples include benzene.
Q7: For a chemical reaction,if <span
Q12: Calculate the pH of the following aqueous
Q19: Calculate E°<sub>cell</sub> for the cell for
Q20: Which of the following compounds is expected
Q28: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q35: For the reaction TlSCN(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q41: Mount Everest rises to a height
Q63: Predict the formula of the product resulting
Q71: Which of the following statements concerning semiconductors
Q71: A mixture of KCl and KClO<sub>3</sub> weighing