The reaction quotient,Q,for a system is 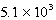 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 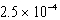
,what will happen as the reaction mixture returns to equilibrium?
Definitions:
Type I Error
The faulted rejection of an authentic null hypothesis, also termed as a "false positive."
Type II Error
The error that occurs when a statistical test fails to reject a false null hypothesis, also known as a "false negative" result.
Statistical Outcome
The result of any single observation or measurement regarding a statistical experiment.
Validity
The extent to which a concept, conclusion or measurement is well-founded and likely corresponds accurately to the real world.
Q2: What is the hydronium-ion concentration in
Q5: Given the following,determine <span class="ql-formula"
Q7: What is the pH of a
Q14: Calcium crystallizes in a face-centered cubic lattice.The
Q23: The thermochemical equation for the formation
Q43: What is responsible for capillary action,a property
Q47: If 25 mL of 0.750 M HCl
Q61: What is the freezing point of a
Q75: Draw the reaction coordinate diagram for an
Q79: What is the pH of a