Nitrogen trifluoride decomposes at to form nitrogen and fluorine gases according to the following equation: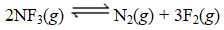
6.00-L reaction vessel is initially charged with 1.96 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0380 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L.atm.mol.K)
Definitions:
Competitive Marketplace
Describes an economic environment where businesses vie for the same customers, offering similar or better goods and services to gain market share.
Increased Choices
A market condition where consumers have a wider range of products or services to choose from, often due to innovation or increased competition.
Monopolies
Describes a market structure in which a single seller dominates the market, facing little to no competition.
Marketers
Professionals engaged in the promotion, distribution, and selling of products or services to consumers or other businesses.
Q20: Which of the following nitrogen oxides are
Q21: If the solubility of O<sub>2</sub> at
Q23: For the formation of 1 mol
Q32: Which of the following cannot be used
Q33: Which of the following solutions has the
Q41: What is the electron geometry around the
Q48: A 50.00-mL solution of 0.0350 M
Q51: Which of the following expressions is not
Q77: If an ionic compound with the formula
Q82: In 1913,the Haber-Bosch process was patented.The product