Excess Ag2SO4(s) is placed in water at 25 C.At equilibrium,the solution contains 0.029 M Ag+(aq) .What is the equilibrium constant for the reaction below?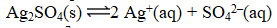
Definitions:
Process Costing System
An accounting methodology used in manufacturing, where costs are assigned to processes or departments and averaged over the units produced, suitable for homogeneous products.
Lumber Mill
A facility where logs are cut, planed, and processed into lumber and other wood products for construction and manufacturing.
Process Cost System
An accounting method used to track costs associated with producing homogenous products in continuous processes.
Work In Process
Inventory that includes goods in the production process but not yet completed, often referred to as WIP.
Q8: For the reaction 2A + B
Q8: When 1.0 mole of acetic acid
Q14: At 700 K,K<sub>p</sub> for the following
Q16: Consider the following half-reactions:<br>Cl<sub>2</sub>(g)+ 2 e<sup>-</sup>
Q23: Point D on the phase diagram is
Q32: Which is NOT an amphiprotic species in
Q39: What is the pH of a
Q56: All of the following species behave as
Q67: Write a balanced half-reaction for the
Q68: A 3.74 gram sample of a certain