At a given temperature,0.0664 mol N2O4(g) is placed in a 1.00 L flask.After reaching equilibrium,the concentration of NO2(g) is 6.1 10-3 M.What is Kc for the reaction below?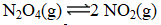
Definitions:
PR Communication
The strategies and processes used by individuals or organizations to manage and disseminate information to the public and the media.
PRSA Code Of Conduct
The ethical guidelines established by the Public Relations Society of America, which its members agree to follow in their professional practices.
Informed Decision Making
The process of making decisions based on relevant information, data, and knowledge to achieve a desired outcome.
Free Flow
The unrestricted movement of information, goods, or people.
Q4: In which of the following reactions does
Q17: A possible mechanism for the gas phase
Q19: A byproduct of biodiesel production is<br>A) methanol<br>B)
Q32: Which of the following cannot be used
Q38: For which of the following compounds is
Q49: According to the Brønsted-Lowry definition,an acid<br>A) increases
Q57: Refer to Diagram 9-1.What is the
Q60: Which one of the following molecules has
Q66: What molar ratio of acetic acid
Q83: If a reaction is third-order with respect