When 0.20 mole HF is dissolved in water to a volume of 1.00 L,5.8% of the HF dissociates to form F-(aq) .What is the equilibrium constant for the reaction?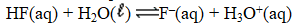
Definitions:
Consumer Preferences
The particular goods and services that consumers demand, reflecting their tastes, desires, and needs.
Economic Shift
A significant change in the condition or direction of an economy, often marked by a variation in key economic indicators.
Production Possibility Frontier
The production possibility frontier is a curve illustrating the maximum feasible amounts of two commodities that a business can produce with its available resources and technology.
Scarce Resources
Natural or human assets available in limited quantities relative to their demand, leading to the necessity of economic allocation.
Q2: Which of the following molecules is expected
Q6: The Henry's law constant for O<sub>2</sub>
Q13: Which of the following statements is/are CORRECT?<br>1)Of
Q31: What is the formula of the common
Q40: Consider the following equilibrium:<br>CO<sub>2</sub>(g)+ H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q45: What is the copper(II)-ion concentration at 25°C
Q45: What is the molar solubility of
Q49: Potassium hydrogen phthalate (KHP)is used to standardize
Q57: In any chemical process,energy must be conserved.This
Q81: The solubility of copper(II)oxalate is 3.2