Given the following chemical equilibria,
N2(g) + O2(g) 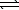 2 NO(g) K1
2 NO(g) K1
N2(g) + 3 H2(g) 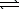 2 NH3(g) K2
2 NH3(g) K2
H2(g) + 1/2 O2(g) 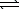 H2O(g) K3
H2O(g) K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g) + 5 O2(g) 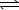 4 NO(g) + 6 H2O(g) K c
4 NO(g) + 6 H2O(g) K c
Definitions:
Barrier Airway Equipment
Devices designed to protect the airway or facilitate breathing in emergency situations or surgical procedures.
Category I Task
A designation for tasks that are considered top priority or utmost importance in a given context.
Standard Precautions
Basic infection prevention practices aimed at reducing the risk of transmission of infectious agents from recognized and unrecognized sources.
Needlestick Prevention
Measures and practices designed to reduce the risk of injury from needles and other sharp medical instruments.
Q3: The vapor pressure of water at 90°C
Q3: In a certain mountain range,water boils at
Q17: Which of the following liquids will be
Q20: For the process Br<sub>2</sub>(l) <span class="ql-formula"
Q38: Which of the following elements is least
Q50: The symbol Q is called the _.
Q71: What is the concentration of Cd<sup>2+</sup>(aq)in
Q80: Which of the following statements concerning a
Q81: The solubility of copper(II)oxalate is 3.2
Q82: For the reaction provided,the rate of