Given the following equilibria,
PbBr2(s) 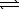 Pb2+(aq) + 2 Br-(aq) K1 = 6.6 0 10-6
Pb2+(aq) + 2 Br-(aq) K1 = 6.6 0 10-6
Pb(OH) 2(s) 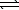 Pb2+(aq) + 2 OH-(aq) K2 = 1.4 10-15
Pb2+(aq) + 2 OH-(aq) K2 = 1.4 10-15
Determine the equilibrium constant,Kc,for the following reaction.
PbBr2(s) + 2 OH-(aq) 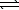 Pb(OH) 2(s) + 2 Br-(aq)
Pb(OH) 2(s) + 2 Br-(aq)
Definitions:
Cross-sectional Study
A research method that analyzes data from a population, or a representative subset, at a specific point in time.
Cognitive Changes
Alterations in cognitive functions such as memory, problem-solving, attention, and language abilities that may occur due to aging, brain injury, or neurological conditions.
Simultaneously
Occurring, done, or existing at the same time.
Cohort Differences
Variations in characteristics or experiences of individuals who are born in different time periods.
Q3: In a certain mountain range,water boils at
Q7: One liter of water is mixed
Q8: In the reaction<br>CuO(s)+ CO<sub>2</sub>(g) <span class="ql-formula"
Q18: Which of the following statements about
Q29: A low melting solid readily dissolves in
Q34: What is the general formula for an
Q46: Which of the following species are likely
Q61: Which equation represents the number of atoms
Q61: Which one of the following sets of
Q67: In HF<sub>2</sub><sup>-</sup> the hydrogen is shared between