The standard enthalpy of formation of ammonia is -46.1 kJ/mol.
1/2 N2(g)+ 3/2 H2(g) 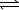 NH3(g)
NH3(g)
Commercially,the reaction is carried out at high temperatures.Using your knowledge of kinetics and equilibrium,explain an advantage and a disadvantage of synthesizing ammonia at high temperatures.
Definitions:
Parental Monitoring
Involves parents overseeing and being aware of their children's activities, whereabouts, and social interactions to guide and protect them.
Stressors
External or internal stimuli that challenge an individual's emotional or physical well-being, requiring them to adapt or respond.
Longitudinal Study
A research design focusing on collecting data from the same subjects repeatedly over a period of time to observe changes or developments.
Incidence of Infanticide
The occurrence rate at which infants are intentionally killed by adults, often influenced by cultural, social, and psychological factors.
Q9: As pure molecular solids,which of the following
Q11: What is the pH of a
Q13: Which of the following statements is INCORRECT?<br>A)
Q29: The conversion (fixation)of N<sub>2</sub> to its compounds
Q31: Because _ particles are relatively large (say,1000
Q36: Which group 1A metal is the strongest
Q48: Use your knowledge of group properties to
Q60: Which two of the following materials are
Q76: The following electrochemical cell has a
Q83: If a reaction is third-order with respect