What is the equilibrium constant for the following reaction,
HCO2H(aq) + CN-(aq) 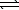 HCO2-(aq) + HCN(aq)
HCO2-(aq) + HCN(aq)
And does the reaction favor the formation of reactants or products? The acid dissociation constant,Ka,for HCO2H is 1.8 10-4 and the acid dissociation constant for HCN is 4.0 10-10.
Definitions:
After Copulation
The period following sexual intercourse or mating in animals, which may involve specific behaviors or physiological processes.
Supply Chain
The interconnected network of individuals, organizations, resources, activities, and technologies involved in the production and sale of a product or service.
Supply Chain Management
The oversight and management of the flow of goods and services, including all processes that transform raw materials into final products.
Observable Behaviour
Actions or conduct that can be directly seen or measured, often used in psychological or organizational analysis.
Q3: Given the initial rate data for
Q25: Which of the following will not as
Q31: Biodiesel is a mixture of<br>A) fats and
Q44: What is the most abundant nitrogen oxide
Q44: What is the OH<sup>-</sup> concentration of
Q59: The lattice energy of NaBr is
Q67: Write a balanced half-reaction for the
Q79: The reaction of NO and O<sub>2</sub>
Q83: Calculate the standard reduction potential for
Q84: Given the initial rate data for