Given the following equilibrium constants,
Ka (HSO4-) = 1.2 10-2
Kb (CH3CO2-) = 5.6 10-10
Kw = 1.00 10-14
Determine the equilibrium constant for the reaction below at 25 C.
HSO4-(aq) + CH3CO2-(aq) 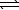 SO42-(aq) + CH3CO2H(aq)
SO42-(aq) + CH3CO2H(aq)
Definitions:
Spiritual Meaning
The significance or purpose ascribed to life or existence based on religious, philosophical, or personal beliefs and practices.
Terminally Ill
A condition in which an individual is facing a disease or condition that is expected to lead to death within a relatively short period of time.
Ineffective Role Performance
A situation where an individual perceives themselves as unable to fulfill the expectations or responsibilities of a particular role.
Stroke
A medical condition where poor blood flow to the brain results in cell death, causing symptoms like paralysis and speech impediments.
Q8: Give two examples of the allotropes of
Q17: The concentration of H<sub>3</sub>O<sup>+</sup> in a
Q18: Which of the following statements about
Q25: All of the following are used by
Q48: What is the K<sub>c</sub> expression for the
Q51: Ammonia,NH<sub>3</sub>,is used as a refrigerant.At its
Q57: In any chemical process,energy must be conserved.This
Q67: Write a balanced half-reaction for the
Q69: Polonium (atomic mass 209.0 g/mol)crystallizes in a
Q81: Gold and platinum are commonly used as