Given the following equilibrium constants,
Ka (HSO4-) = 1.2 10-2
Kb (CH3CO2-) = 5.6 10-10
Kw = 1.00 10-14
Determine the equilibrium constant for the reaction below at 25 C.
HSO4-(aq) + CH3CO2-(aq) 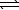 SO42-(aq) + CH3CO2H(aq)
SO42-(aq) + CH3CO2H(aq)
Definitions:
Menopause
Menopause is a natural biological process that marks the end of a woman's reproductive years, typically occurring in the late 40s to early 50s.
Physical Discomfort
A state of physical unease or pain which can range from mild to severe, often indicating an underlying condition or ailment.
Pregnant
is the state of carrying a developing embryo or fetus within the female body, typically lasting around nine months in humans.
Selection Effect
A statistical bias that occurs when the sample chosen for analysis is not representative of the population intended to be analyzed.
Q25: At a given temperature,K = 0.021 for
Q28: What is the mole fraction of urea,CH<sub>4</sub>N<sub>2</sub>O,in
Q39: Xenon has an enthalpy of vaporization
Q48: Calculate E<sub>cell</sub> for the following electrochemical
Q51: Ammonia,NH<sub>3</sub>,is used as a refrigerant.At its
Q63: Calculate the activation energy,E<sub>a</sub>,for<br>N<sub>2</sub>O<sub>5</sub>(g) <span class="ql-formula"
Q66: On a relative basis,the weaker the intermolecular
Q72: The rate law for a reaction
Q73: Niobium crystallizes in a body-centered cubic lattice.If
Q75: The rate of effusion of an unknown