Which of the following reactions would be expected to have a positive entropy change, ∆rS° > 0?
1. 2 SO2(g) + O2(g) → 2 SO3(g)
2. Ba(OH) 2(s) → BaO(s) + H2O(g)
3. CO(g) + 2 H2(g) CH3OH( 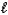 )
)
Definitions:
Voluminous
Describing something as having great volume or size, or being very lengthy or detailed.
Passive Listening
Listening to someone without giving active feedback, often resulting in missing important details or nuances.
Acceptance
The act of receiving or agreeing to something offered or proposed with a positive attitude.
Extra Hours
Additional hours worked beyond the usual or scheduled working time, often eligible for overtime compensation.
Q11: If a cube of ice at
Q12: A(n)_ reaction is one where an element
Q12: If the concentration of sodium carbonate
Q18: The solubility product equilibrium constant,K<sub>sp</sub>,of silver
Q50: The symbol Q is called the _.
Q59: What is the maximum hydroxide-ion concentration
Q70: The carbon-carbon bonding of cyclobutane is shown
Q76: What is the name of the following
Q85: Cis-1,2-dichloroethylene and trans-1,2-dichloroethylene are examples of _
Q87: Reduction of a ketone produces a(n)_.<br>A) alkane<br>B)